Worldwide, some 240 million
people have chronic hepatitis B virus (HBV), with the highest rates of
infection in Africa and Asia. Our understanding of the natural history of HBV
infection and the potential for therapy of the resultant disease is
continuously improving. New data have become available since the previous APASL
guidelines for management of HBV infection were published in 2012.
The
objective of this manuscript is to update the recommendations for the optimal
management of chronic HBV infection. The 2015 guidelines were developed by a
panel of Asian experts chosen by the APASL. The clinical practice guidelines
are based on evidence from existing publications or, if evidence was
unavailable, on the experts’ personal experience and opinion after
deliberations. Manuscripts and abstracts of important meetings published
through January 2015 have been evaluated.
This guideline covers the full
spectrum of care of patients infected with hepatitis B, including new
terminology, natural history, screening, vaccination, counseling, diagnosis,
assessment of the stage of liver disease, the indications, timing, choice and
duration of single or combination of antiviral drugs, screening for HCC,
management in special situations like childhood, pregnancy, coinfections, renal
impairment and pre- and post-liver transplant, and policy guidelines.
However,
areas of uncertainty still exist, and clinicians, patients, and public health
authorities must therefore continue to make choices on the basis of the
evolving evidence. The final clinical practice guidelines and recommendations
are presented here, along with the relevant background information.
Below: Treatment indications for chronic HBV-infected patients with cirrhosis or reactivation of chronic HBV infection
Below: Treatment indications for chronic HBV-infected patients with cirrhosis or reactivation of chronic HBV infection
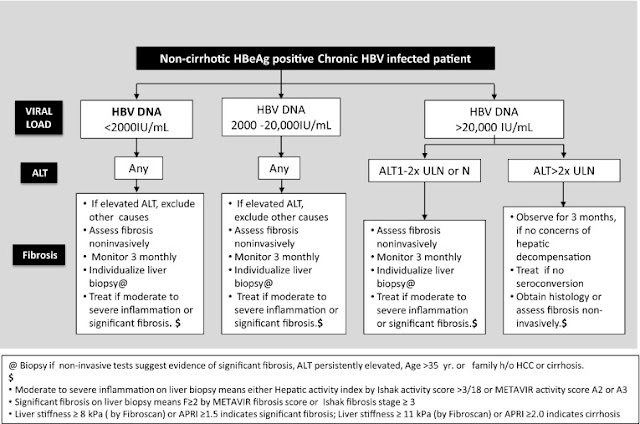
Below: Treatment indications for noncirrhotic HBeAg-negative chronic HBV-infected patients
Below: Reverse transcriptase mutations associated with drug resistance
Below: Cumulative incidence of antiviral resistance in long-term studies of NA therapy
Below: Treatment of CHB infection in HIV infected individuals
Below: Treatment of HBV–HCV coinfected patients
Recommendations for infected persons regarding prevention of transmission of HBV to others
Below: Reverse transcriptase mutations associated with drug resistance
Below: Cumulative incidence of antiviral resistance in long-term studies of NA therapy
Below: Treatment of CHB infection in HIV infected individuals
Below: Treatment of HBV–HCV coinfected patients
Recommendations for infected persons regarding prevention of transmission of HBV to others
| Have sexual contacts vaccinated |
| Use barrier protection during sexual intercourse if partner not vaccinated or naturally immune |
| Do not share toothbrushes or razors |
| Cover open cuts and scratches |
| Clean blood spills with detergent or bleach |
| Do not donate blood, organs or sperm |
| Can participate in all activities including contact sports |
| Children should not be excluded from daycare or school participation and should not be isolated from other children |
| Can share food, utensils, or kiss others |
By:  M. Kumar, G. K. Lau, Z. Abbas, H. L. Y. Chan, C. J. Chen, D. S. Chen, H. L. Chen, P. J. Chen, R. N. Chien, A. K. Dokmeci, Ed Gane, J. L. Hou, W. Jafri, J. Jia, J. H. Kim, C. L. Lai, H. C. Lee, S. G. Lim, C. J. Liu, S. Locarnini, M. Al Mahtab, R. Mohamed, M. Omata, J. Park, T. Piratvisuth, B. C. Sharma, J. Sollano, F. S. Wang, L. Wei, M. F. Yuen, S. S. Zheng, and J. H. Kao
M. Kumar, G. K. Lau, Z. Abbas, H. L. Y. Chan, C. J. Chen, D. S. Chen, H. L. Chen, P. J. Chen, R. N. Chien, A. K. Dokmeci, Ed Gane, J. L. Hou, W. Jafri, J. Jia, J. H. Kim, C. L. Lai, H. C. Lee, S. G. Lim, C. J. Liu, S. Locarnini, M. Al Mahtab, R. Mohamed, M. Omata, J. Park, T. Piratvisuth, B. C. Sharma, J. Sollano, F. S. Wang, L. Wei, M. F. Yuen, S. S. Zheng, and J. H. Kao
 M. Kumar, G. K. Lau, Z. Abbas, H. L. Y. Chan, C. J. Chen, D. S. Chen, H. L. Chen, P. J. Chen, R. N. Chien, A. K. Dokmeci, Ed Gane, J. L. Hou, W. Jafri, J. Jia, J. H. Kim, C. L. Lai, H. C. Lee, S. G. Lim, C. J. Liu, S. Locarnini, M. Al Mahtab, R. Mohamed, M. Omata, J. Park, T. Piratvisuth, B. C. Sharma, J. Sollano, F. S. Wang, L. Wei, M. F. Yuen, S. S. Zheng, and J. H. Kao
M. Kumar, G. K. Lau, Z. Abbas, H. L. Y. Chan, C. J. Chen, D. S. Chen, H. L. Chen, P. J. Chen, R. N. Chien, A. K. Dokmeci, Ed Gane, J. L. Hou, W. Jafri, J. Jia, J. H. Kim, C. L. Lai, H. C. Lee, S. G. Lim, C. J. Liu, S. Locarnini, M. Al Mahtab, R. Mohamed, M. Omata, J. Park, T. Piratvisuth, B. C. Sharma, J. Sollano, F. S. Wang, L. Wei, M. F. Yuen, S. S. Zheng, and J. H. Kao
Department of
Hepatology, Institute of Liver and Biliary Sciences, New Delhi, India
Division of
Gastroenterology and Hepatology, Humanity and Health Medical Centre, Hong Kong
SAR, China
Department of
Hepatogastroenterlogy, Sindh Institute of Urology and Transplantation, Karachi,
Pakistan
Institute of
Digestive Disease, The Chinese University of Hong Kong, Hong Kong, China
Genomics Research
Center, Academia Sinica, National Taiwan University, Taipei, Taiwan
Department of
Internal Medicine, National Taiwan University College of Medicine, Taipei,
Taiwan
Graduate
Institute of Clinical Medicine, National Taiwan University College of Medicine,
Taipei, Taiwan
Department of
Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Liver Research
Unit, Chang Gung Memorial Hospital and University, Chilung, Taiwan
Department of
Gastroenterology, Ankara University School of Medicine, Ankara, Turkey
New Zealand Liver
Transplant Unit, Auckland City Hospital, Auckland, New Zealand
Department of
Infectious Diseases and Hepatology Unit, Nanfang Hospital, Guangzhou, China
Department of
Medicine, Aga Khan University, Karachi, Pakistan
Beijing
Friendship Hospital, Capital Medical University, Beijing, China
Seoul, Korea
Department of
Medicine, University of Hong Kong, Hong Kong, China
Internal Medicine
Asan Medical Center, Seoul, Korea
Division of
Gastroenterology and Hepatology, National University Health System, Singapore,
Singapore
Research and
Molecular Development, Victorian Infectious Diseases Reference Laboratory,
Melbourne, Australia
Bangabandhu
Sheikh Mujib Medical University, Dhaka, Bangladesh
Department of
Medicine, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia
Yamanashi
Hospitals (Central and Kita) Organization, 1-1-1 Fujimi, Kofu-shi, Yamanashi
400-8506 Japan
Department of
Internal Medicine, Institute of Gastroenterology, Yonsei University College of
Medicine, Seoul, Korea
NKC Institute of
Gastroenterology and Hepatology, Prince of Songkla University, Songkhla,
Thailand
Department of
Gastroenterology, G.B. Pant Hospital, New Delhi, India
Department of
Medicine, University of Santo Tomas, Manila, Philippines
The Institute of
Translational Hepatology, Beijing, China
Treatment and
Research Center for Infectious Diseases, Beijing 302 Hospital, Beijing, China
Peking University
Hepatology Institute, Beijing, China
Division of
Gastroenterology and Hepatology, Department of Medicine, University of Hong
Kong, Pofulam, Hong Kong
Department of
Hepatobiliary and Pancreatic Surgery, Collaborative Innovation Center for
Diagnosis and Treatment of Infectious Diseases, Key Laboratory of Combined
Multi-organ Transplantation, Ministry of Public Health, First Affiliated
Hospital, Zhejiang University School of Medicine, Hangzhou, 310003 Zhejiang
Province China
Graduate
Institute of Clinical Medicine and Hepatitis Research Center, National Taiwan
University College of Medicine, National Taiwan University Hospital, Taipei,
Taiwan
S. K. Sarin, Email: moc.liamg@nirasvihs.
More at: https://twitter.com/hiv insight








No comments:
Post a Comment