BACKGROUND:
WHO
recommends regular viral load (VL) monitoring of patients on antiretroviral
therapy (ART) for timely detection of virological failure, prevention of
acquired HIV drug resistance (HIVDR) and avoiding unnecessary switching to
second-line ART. However, the cost and complexity of routine VL testing remains
prohibitive in most resource limited settings (RLS). We evaluated a simple,
low-cost, qualitative viral-failure assay (VFA) on dried blood spots (DBS) in
three clinical settings in Uganda.
METHODS:
We
conducted a cross-sectional diagnostic accuracy study in three HIV/AIDS
treatment centres at the Joint Clinical Research Centre in Uganda. The VFA
employs semi-quantitative detection of HIV-1 RNA amplified from the LTR gene.
We used paired dry blood spot (DBS) and plasma with the
COBASAmpliPrep/COBASTaqMan, Roche version 2 (VLref) as the reference assay. We
used the VFA at two thresholds of viral load, (>5,000 or >1,000
copies/ml).
RESULTS:
496
paired VFA and VLref results were available for comparative analysis. Overall,
VFA demonstrated 78.4% sensitivity, (95% CI: 69.7%-87.1%), 93% specificity (95%
CI: 89.7%-96.4%), 89.3% accuracy (95% CI: 85%-92%) and an agreement kappa =
0.72 as compared to the VLref. The predictive values of positivity and negativity
among patients on ART for >12 months were 72.7% and 99.3%, respectively.
CONCLUSIONS:
VFA
allowed 89% of correct classification of VF. Only 11% of the patients were
misclassified with the potential of unnecessary or late switch to second-line
ART. Our findings present an opportunity to roll out simple and affordable VL
monitoring for HIV-1 treatment in RLS.
Below: Receiver Operating Characteristics Curve for VFA compared to Cobas-Ampliprep
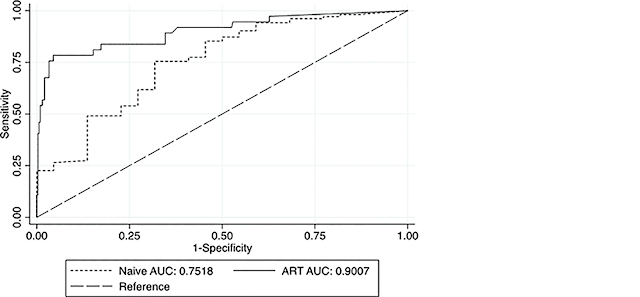
Below: Receiver Operating Characteristics Curve for VFA comparing ART–naïve and treated PLWHAs (VL threshold ≥ 5,000 cp/mL)
By: Balinda SN1, Ondoa P2, Obuku EA1, Kliphuis A3, Egau I1, Bronze M4, Kasambula L1, Schuurman R5, Spieker N3, Rinke de Wit TF2, Kityo C1; ART–A consortium.
- 1Joint Clinical Research Center, P.O. Box 10005, Kampala, Uganda.
- 2Amsterdam Institute for Global Health and Development, Department of Global Health, Academic medical Center, Trinity C Building, Pietersbergweg 17, 1105 BM, Amsterdam, the Netherlands.
- 3PharmAccess International, Amsterdam, Trinity Building C Pietersbergweg 17, 1105 BM, Amsterdam, the Netherlands.
- 4Wits Health Consortium, University of the Witwatersrand, 1 Jan Smuts Avenue, Braamfontein 2000, Johannesburg, South Africa.
- 5University Medical Centre Utrecht, P.O. Box 80125, 3508 TC, Utrecht, the Netherlands.
- PLoS One. 2016 Jan 29;11(1):e0145110. doi: 10.1371/journal.pone.0145110. eCollection 2016.



No comments:
Post a Comment